4.7 Entropy on a Microscopic Scale
Learning Objectives
By the end of this section, you will be able to:
- Interpret the meaning of entropy at a microscopic scale
- Calculate a change in entropy for an irreversible process of a system and contrast with the change in entropy of the universe
- Explain the third law of thermodynamics
We have seen how entropy is related to heat exchange at a particular temperature. In this section, we consider entropy from a statistical viewpoint. Although the details of the argument are beyond the scope of this textbook, it turns out that entropy can be related to how disordered or randomized a system is—the more it is disordered, the higher is its entropy. For example, a new deck of cards is very ordered, as the cards are arranged numerically by suit. In shuffling this new deck, we randomize the arrangement of the cards and therefore increase its entropy (Figure 4.17). Thus, by picking one card off the top of the deck, there would be no indication of what the next selected card will be.

The second law of thermodynamics requires that the entropy of the universe increase in any irreversible process. Thus, in terms of order, the second law may be stated as follows:
In any irreversible process, the universe becomes more disordered. For example, the irreversible free expansion of an ideal gas, shown in Figure 4.2, results in a larger volume for the gas molecules to occupy. A larger volume means more possible arrangements for the same number of atoms, so disorder is also increased. As a result, the entropy of the gas has gone up. The gas in this case is a closed system, and the process is irreversible. Changes in phase also illustrate the connection between entropy and disorder.
Example 4.7
Entropy Change of the Universe
Suppose we place 50 g of ice at in contact with a heat reservoir at . Heat spontaneously flows from the reservoir to the ice, which melts and eventually reaches a temperature of . Find the change in entropy of (a) the ice and (b) the universe.Strategy
Because the entropy of a system is a function of its state, we can imagine two reversible processes for the ice: (1) ice is melted at and (2) melted ice (water) is warmed up from to under constant pressure. Then, we add the change in entropy of the reservoir when we calculate the change in entropy of the universe.Solution
- From Equation 4.10, the increase in entropy of the ice is
- During this transition, the reservoir gives the ice an amount of heat equal to
This leads to a change (decrease) in entropy of the reservoir:
The increase in entropy of the universe is therefore
Significance
The entropy of the universe therefore is greater than zero since the ice gains more entropy than the reservoir loses. If we considered only the phase change of the ice into water and not the temperature increase, the entropy change of the ice and reservoir would be the same, resulting in the universe gaining no entropy.This process also results in a more disordered universe. The ice changes from a solid with molecules located at specific sites to a liquid whose molecules are much freer to move. The molecular arrangement has therefore become more randomized. Although the change in average kinetic energy of the molecules of the heat reservoir is negligible, there is nevertheless a significant decrease in the entropy of the reservoir because it has many more molecules than the melted ice cube. However, the reservoir’s decrease in entropy is still not as large as the increase in entropy of the ice. The increased disorder of the ice more than compensates for the increased order of the reservoir, and the entropy of the universe increases by 4.6 J/K.
You might suspect that the growth of different forms of life might be a net ordering process and therefore a violation of the second law. After all, a single cell gathers molecules and eventually becomes a highly structured organism, such as a human being. However, this ordering process is more than compensated for by the disordering of the rest of the universe. The net result is an increase in entropy and an increase in the disorder of the universe.
Check Your Understanding 4.5
In Example 4.7, the spontaneous flow of heat from a hot object to a cold object results in a net increase in entropy of the universe. Discuss how this result can be related to an increase in disorder of the system.
The second law of thermodynamics makes clear that the entropy of the universe never decreases during any thermodynamic process. For any other thermodynamic system, when the process is reversible, the change of the entropy is given by . But what happens if the temperature goes to zero, ? It turns out this is not a question that can be answered by the second law.
A fundamental issue still remains: Is it possible to cool a system all the way down to zero kelvin? We understand that the system must be at its lowest energy state because lowering temperature reduces the kinetic energy of the constituents in the system. What happens to the entropy of a system at the absolute zero temperature? It turns out the absolute zero temperature is not reachable—at least, not though a finite number of cooling steps. This is a statement of the third law of thermodynamics, whose proof requires quantum mechanics that we do not present here. In actual experiments, physicists have continuously pushed that limit downward, with the lowest temperature achieved at about in a low-temperature lab at the Helsinki University of Technology in 2008.
Like the second law of thermodynamics, the third law of thermodynamics can be stated in different ways. One of the common statements of the third law of thermodynamics is: The absolute zero temperature cannot be reached through any finite number of cooling steps.
In other words, the temperature of any given physical system must be finite, that is, . This produces a very interesting question in physics: Do we know how a system would behave if it were at the absolute zero temperature?
The reason a system is unable to reach 0 K is fundamental and requires quantum mechanics to fully understand its origin. But we can certainly ask what happens to the entropy of a system when we try to cool it down to 0 K. Because the amount of heat that can be removed from the system becomes vanishingly small, we expect that the change in entropy of the system along an isotherm approaches zero, that is,
This can be viewed as another statement of the third law, with all the isotherms becoming isentropic, or into a reversible ideal adiabat. We can put this expression in words: A system becomes perfectly ordered when its temperature approaches absolute zero and its entropy approaches its absolute minimum.
The third law of thermodynamics puts another limit on what can be done when we look for energy resources. If there could be a reservoir at the absolute zero temperature, we could have engines with efficiency of , which would, of course, violate the second law of thermodynamics.
Example 4.8
Entropy Change of an Ideal Gas in Free Expansion
An ideal gas occupies a partitioned volume inside a box whose walls are thermally insulating, as shown in Figure 4.18(a). When the partition is removed, the gas expands and fills the entire volume of the box, as shown in part (b). What is the entropy change of the universe (the system plus its environment)?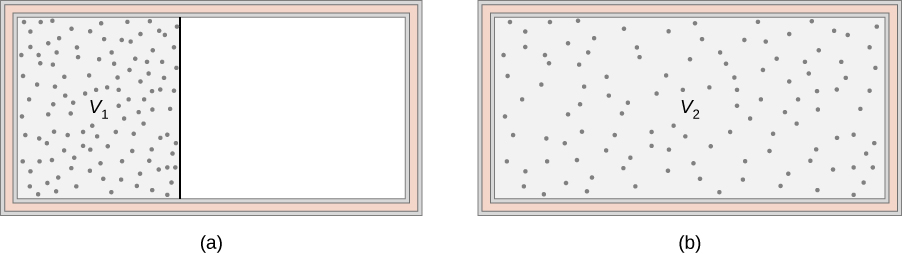
Strategy
The adiabatic free expansion of an ideal gas is an irreversible process. There is no change in the internal energy (and hence temperature) of the gas in such an expansion because no work or heat transfer has happened. Since the entropy of the system is a state variable, the entropy change of the system during the irreversible process is the same as for any other path. A convenient alternative reversible path connecting the same two equilibrium states for which we can calculate the change in entropy is a slow (quasistatic), isothermal expansion from to . In this process, the gas could be expanding against a piston while in thermal contact with a heat reservoir, as in step 1 of the Carnot cycle.Solution
Since the temperature is constant, the entropy change is given by wherebecause Now, with the help of the ideal gas law, we have
so the change in entropy of the gas is
Because , is positive, and the entropy of the gas has gone up during the free expansion.
Significance
What about the environment? The walls of the container are thermally insulating, so no heat exchange takes place between the gas and its surroundings. The entropy of the environment is therefore constant during the expansion. The net entropy change of the universe is then simply the entropy change of the gas. Since this is positive, the entropy of the universe increases in the free expansion of the gas.Example 4.9
Entropy Change during Heat Transfer
Heat flows from a steel object of mass 4.00 kg whose temperature is 400 K to an identical object at 300 K. Assuming that the objects are thermally isolated from the environment, what is the net entropy change of the universe after thermal equilibrium has been reached?Strategy
Since the objects are identical, their common temperature at equilibrium is 350 K. To calculate the entropy changes associated with their transitions, we substitute the irreversible process of the heat transfer by two isobaric, reversible processes, one for each of the two objects. The entropy change for each object is then given bySolution
Using , the specific heat of steel, we have for the hotter objectSimilarly, the entropy change of the cooler object is
The net entropy change of the two objects during the heat transfer is then
Significance
The objects are thermally isolated from the environment, so its entropy must remain constant. Thus, the entropy of the universe also increases by 37 J/K.Check Your Understanding 4.6
A quantity of heat Q is absorbed from a reservoir at a temperature by a cooler reservoir at a temperature What is the entropy change of the hot reservoir, the cold reservoir, and the universe?
Check Your Understanding 4.7
A 50-g copper piece at a temperature of is placed into a large insulated vat of water at . (a) What is the entropy change of the copper piece when it reaches thermal equilibrium with the water? (b) What is the entropy change of the water? (c) What is the entropy change of the universe?
Interactive
View this site to learn about entropy and microstates. Start with a large barrier in the middle and 1000 molecules in only the left chamber. What is the total entropy of the system? Now remove the barrier and let the molecules travel from the left to the right hand side? What is the total entropy of the system now? Lastly, add heat and note what happens to the temperature. Did this increase entropy of the system?